UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): January 7, 2019
Solid Biosciences Inc.
(Exact Name of Registrant as Specified in Charter)
| Delaware | 001-38360 | 90-0943402 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
141 Portland Street, Fifth Floor
Cambridge, MA 02139
(Address of Principal Executive Offices) (Zip Code)
Registrant’s telephone number, including area code: (617) 337-4680
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
| Item 7.01. | Regulation FD Disclosure. |
On January 9, 2019, Solid Biosciences Inc. (the “Company”) intends to make a slide presentation at the 37th Annual J.P. Morgan Healthcare Conference. Beginning on January 8, 2019, the Company also plans to use the presentation in meetings with investors. A form of the slide presentation is being furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information responsive to Item 7.01 of this Form 8-K, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
| Item 9.01. | Financial Statements and Exhibits. |
| (d) | Exhibits: |
99.1 Form of Presentation of Solid Biosciences Inc., dated January 8, 2019
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| SOLID BIOSCIENCES INC. | ||||||
| Date: January 7, 2019 | By: | /s/ Jennifer Ziolkowski | ||||
| Name: Jennifer Ziolkowski | ||||||
| Title: Chief Financial Officer | ||||||

Solid Biosciences Ilan Ganot, Co-Founder, CEO and President January 2019 Exhibit 99.1

Forward-Looking Statements This presentation includes “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, which involve a number of risks and uncertainties. These forward-looking statements include all matters that are not historical facts and, without limiting the foregoing, can be identified by the use of forward-looking terminology, including the terms “believe,” “estimate,” “project,” “anticipate,” “expect,” “seek,” “predict,” “continue,” “possible,” “intend,” “may,” “might,” “will,” “could,” would” or “should” or, in each case, their negative, or other variations or comparable terminology. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs or current expectations concerning, among other things, our product candidates, research and development and clinical trial plans, manufacturing plans, commercialization objectives, prospects, strategies, the industry in which we operate and potential collaborations. We derive many of our forward-looking statements from our operating budgets and forecasts, which are based upon many detailed assumptions. While we believe that our assumptions are reasonable, we caution that it is very difficult to predict the impact of known factors, and, of course, it is impossible for us to anticipate all factors that could affect our actual results. For a discussion of potential risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the “Risk Factors” section, as well as discussions of potential risks, uncertainties and other important factors, in our most recent filings with the Securities and Exchange Commission. All forward-looking statements included in this presentation represent our views as of the date hereof and should not be relied upon as representing our views as of any date subsequent to the date on the cover page of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we specifically disclaim any obligation to do so. No representation or warranty is made as to the accuracy or completeness of the information or analysis in this presentation.

Purpose-Built to Solve Duchenne Muscular Dystrophy © 2019 Solid Biosciences 360-degree Approach Address all facets of DMD Differentiated Lead Gene Transfer Preliminary clinical data this quarter Scalable Manufacturing Process Meet clinical and commercial needs

Duchenne Is A Devastating Muscle-Wasting Disease 10-15,000 cases in the U.S. *Source: Erik Landfeldt, Peter Lindgren, Christopher F. Bell, et al. The burden of Duchenne muscular dystrophy: An international, cross-sectional study. Neurology 2014. Progressive & irreversible Caused by mutations in the dystrophin gene Economic burden in U.S.* No good treatment options
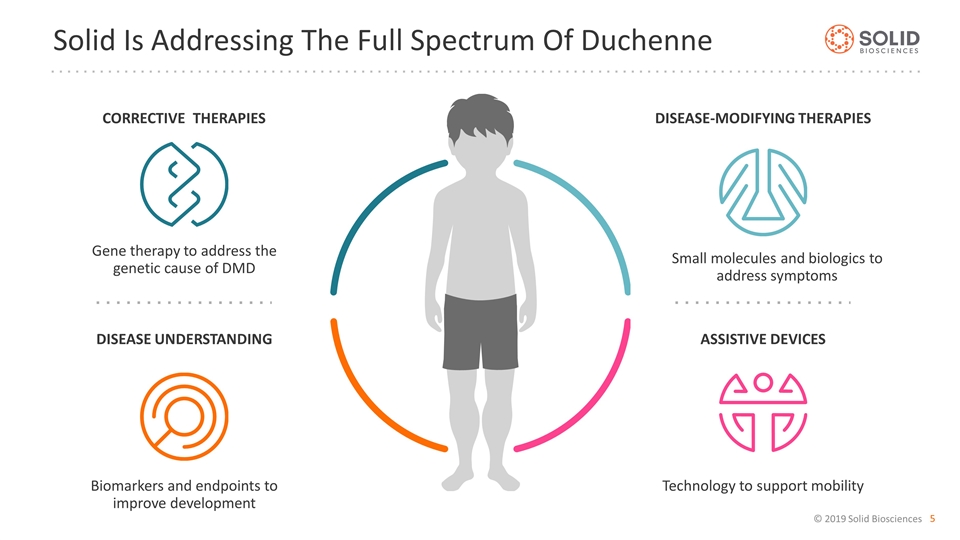
Solid Is Addressing The Full Spectrum Of Duchenne Corrective Therapies Gene therapy to address the genetic cause of DMD Disease understanding Assistive devices Biomarkers and endpoints to improve development Technology to support mobility Disease-modifying therapies Small molecules and biologics to address symptoms

Solid Pipeline PRECLINICAL PHASE I/II SGT-001 Gene Transfer Anti-LTBP4 Biologic MARKETED Corrective Therapies Disease Modifying Therapies DISCOVERY NAME PHASE III Solid Suit PROTOTYPE DEVELOPMENT TESTING Assistive Devices MARKETED NAME Next-Generation Promoter Next-Generation Vector Next-Generation Delivery Muscle Strength RESEARCH DISCOVERY PRECLINICAL EXPLORATORY PRE-DEVELOPMENT PROGRAMS

Innovation in Gene Transfer Corrective Therapies

Gene Therapy To Address The Genetic Cause Of DMD Visual representation only Healthy muscle Muscle Membrane Myofibril Actin Dystrophin Dystrophin protects the muscle from damage and stabilizes critical dystrophin-associated proteins

Gene Therapy To Address The Genetic Cause Of DMD Visual representation only Dystrophic muscle In DMD, mutations in the dystrophin gene result in the loss of functional dystrophin protein Muscle fibers become unstable, lose the ability to repair and become fibrotic Myofibril Muscle Membrane Actin

Gene Therapy To Address The Genetic Cause Of DMD Visual representation only Treated muscle Myofibril Microdystrophin Microdystrophin gene transfer encodes for a functional dystrophin protein surrogate designed to replace the missing dystrophin protein Actin Muscle Membrane

Each Component Of SGT-001 Was Carefully Selected AAV9 Vector Skeletal and cardiac transduction CK8 Promoter Expression is highly targeted SGT-001 microdystrophin Transgene Restore key functions of a complex protein

SGT-001 Microdystrophin Has A Differentiated Composition Visual representation only Full Length Dystrophin Protein SGT-001 Microdystrophin Protein Exclusive licenses to key patent portfolios covering microdystrophin variants and functional domains (e.g. the nNOS binding domain) SGT-001 selection based on more than 30 years of research; confirmed through internal comparative analysis COOH OH

SGT-001 Promotes Significant Cardiac And Skeletal Muscle Microdystrophin Expression In Mice Normal Dystrophic 5E13 vg/kg 2E14 vg/kg Heart Skeletal Muscle (Quadriceps) SGT-001 Solid Biosciences data on file.

CK8 Muscle-Specific Promoter Restricts Expression To Muscles Solid Biosciences data on file. Three month efficacy study in GRMD canines. Representative only. Non-target Tissue Target Tissue 100% 50% 25% 10% 0% 100% 50% 25% 10% 0% 100% 50% 25% 10% 0% Dystrophin Dystrophin Dystrophin Microdystrophin Microdystrophin Microdystrophin SGT-001 Treated Diaphragm SGT-001 Treated Liver SGT-001 Treated Vastus Lateralis

Microdystrophin With nNOS Binding Domain Selected Based On Extensive Comparative Analysis nNOS Negative nNOS Positive (SGT-001) Vehicle Wild Type J. Chamberlain et al. submitted for publication.

Significant Functional Benefit Demonstrated In Dystrophic Canines Solid Biosciences data on file. Three month efficacy study in GRMD canines. N=3 per group | * p<0.05 statistical significance.

Long-term Durability Observed In Canines 30 Months 1 Month 3 Months 24 Months Hakim et al. American Society of Gene and Cell Therapy (ASGCT) 2018.

IGNITE DMD SGT-001 Clinical Program

SGT-001 Phase I/II Clinical Study Ongoing Ambulatory children and non-ambulatory adolescents aged 4-17 (n=16-32) SGT-001 treatment group Starting at 5E13 vg/kg; plan to escalate dose Observation Matched control group SGT-001 Primary Endpoints: Safety SGT-001 microdystrophin expression Secondary Endpoints: Muscle function and strength Cardiac and respiratory function Muscle mass area and composition (MRI) 12 month primary endpoint

Early Planning Enabled A Rational Clinical Approach Designed to obtain efficacy data at multiple dose levels Microdystrophin expression, muscle function and dose response Includes randomized control arm for comparative data Multiple patients and their matched controls enrolled Supports evaluation across a broad population Patients of different ages and stages of disease progression Offers flexibility to assess kinetics of microdystrophin expression over time Biopsies at baseline, 12 months and intermediate timepoint (45 days; 3, 6 or 9 months) Will enable productive conversations with regulators Preliminary data anticipated in Q1 2019 Microdystrophin expression via western blot and immunofluorescence nNOS localization β-Sarcoglycan localization Biodistribution Supportive data, as appropriate

Producing Materials Manufacturing

Addressing The DMD Gene Therapy Supply Challenge HIGH PREVALENCE ( ) ( ) ( ) HIGH AVERAGE PATIENT WEIGHT HIGH DOSES SIGNIFICANT SUPPLY NEEDS Move quickly with a process that scales up to meet the needs of all patients with DMD Solid Manufacturing Strategy © 2019 Solid Biosciences

Successfully scaled up to 250L in suspension and produced multiple batches Each 250L batch can dose multiple patients Utilizes proven, validated and widely-available standard bioreactors GMP Manufacturing Process Currently Producing At Significant Volume 250 Liter Successful scale up to 250L suspension complete Commercial scale Further scale if needed 2,000 Liter 500+ Liter 50 Liter 25 Liter 2L CellSTACK® HYPERStack®
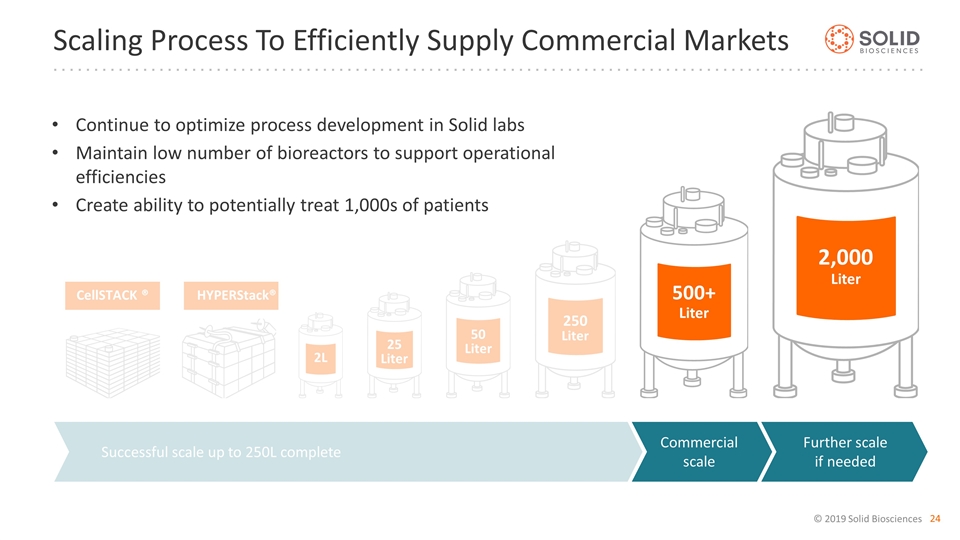
Continue to optimize process development in Solid labs Maintain low number of bioreactors to support operational efficiencies Create ability to potentially treat 1,000s of patients Scaling Process To Efficiently Supply Commercial Markets 250 Liter Successful scale up to 250L complete Commercial scale Further scale if needed 2,000 Liter 500+ Liter 50 Liter 25 Liter 2L CellSTACK ® HYPERStack®

Expanding Pipeline LTBP4 and Next Generation Gene Therapies

Anti-LTBP4 Disease-Modifying Therapy To Address Fibrosis Age at loss of ambulation (yrs.) 5 10 15 100 80 60 40 20 0 Percent ambulatory Improved Muscle Function Decreased Muscle Fibrosis Positive results from blinded, 24-week efficacy study LTBP4 is a powerful genetic modifier in DMD* mdx/hLTBP4 mice, dosed weekly x 24wks (Demonbreun, Quattrocelli, McNally – unpublished data) Ikaika Therapeutics *Flanigan et al. Annals of Neurology. 2013.

Internal And Partnered Programs To Build Comprehensive Pipeline For Duchenne Augment Muscle Strength Add Functionality to the Microdystrophin Transgene Expand Population and Enable Re-administration Enhance Tissue Expression
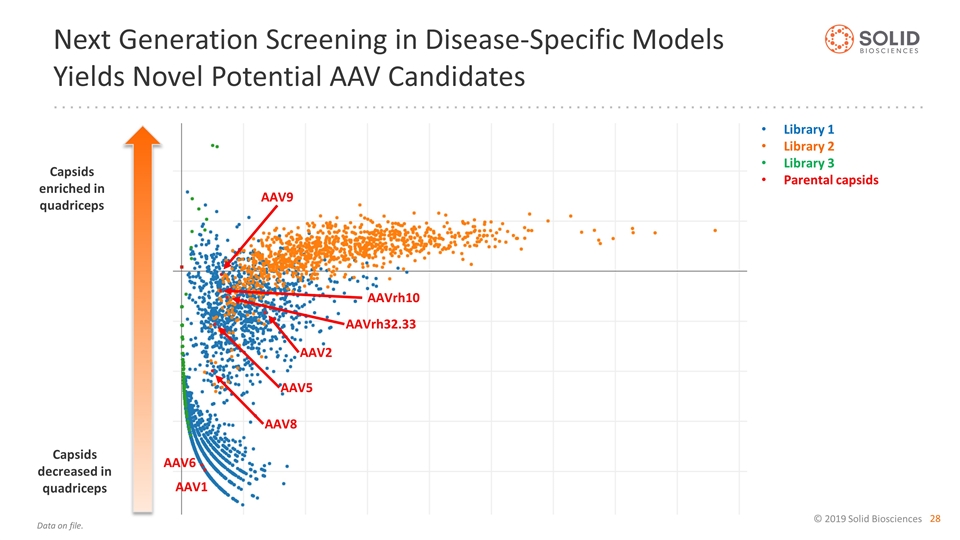
Next Generation Screening in Disease-Specific Models Yields Novel Potential AAV Candidates Library 1 Library 2 Library 3 Parental capsids AAV9 AAVrh32.33 AAV2 AAV5 AAV8 AAVrh10 AAV6 AAV1 Capsids enriched in quadriceps Capsids decreased in quadriceps Data on file.

Program Advancement Preliminary data in the first quarter of 2019 Interim analysis in the second half of 2019 Pipeline Progress LTBP4 program toward IND Advance next generation promoters/vectors Support mission with targeted business development Significant Catalysts In 2019 Manufacturing process development and scale up Regulatory discussions to define approval path SGT-001 Clinical Data
